1.2 M
1.2 M - For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. Always add acid to water slowly and with stirring. He saw what a strenuous and complicated it was to. In 1 ml of 0.990 m cuso4 solution, there are 0.990 moles of cuso4 present. To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final. What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? To prepare a 0.100 m hcl solution from a 1.50 m hcl solution, you need to use the dilution. Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen.
Always add acid to water slowly and with stirring. What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? He saw what a strenuous and complicated it was to. In 1 ml of 0.990 m cuso4 solution, there are 0.990 moles of cuso4 present. Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen. To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final. To prepare a 0.100 m hcl solution from a 1.50 m hcl solution, you need to use the dilution. For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water.
What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. Always add acid to water slowly and with stirring. In 1 ml of 0.990 m cuso4 solution, there are 0.990 moles of cuso4 present. Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen. He saw what a strenuous and complicated it was to. To prepare a 0.100 m hcl solution from a 1.50 m hcl solution, you need to use the dilution. To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final.
👏👏👏
He saw what a strenuous and complicated it was to. Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen. For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. To prepare.
104587407_p0.png SM.MS Simple Free Image Hosting
Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen. For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. What volume of a 1.50 m hcl solution should you use to.
Fendress Inflatable fenders
Always add acid to water slowly and with stirring. What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. In 1 ml of.
Redesigned Clover r/ZeroEscape
What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen. For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of.
Let’s give a round of applause to the fierce woman who was right all
For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final. Pascal had.
Summer classes just started. Guess my major 💻
Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen. Always add acid to water slowly and with stirring. For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. In 1 ml.
Photo posted by Regina Asaba (iamreginaasaba)
What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final. To prepare a 0.100 m hcl solution.
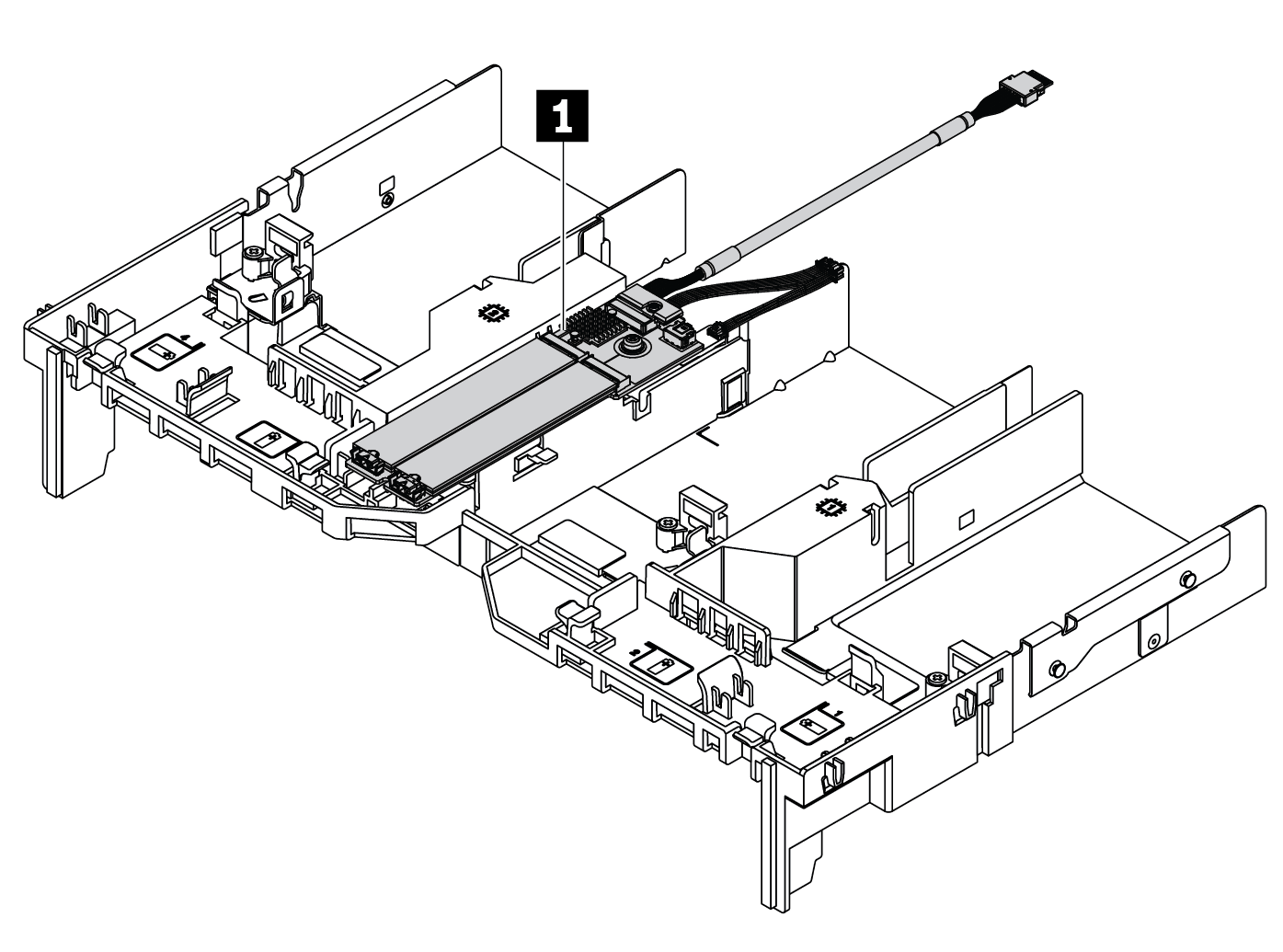
การเปลี่ยนอะแดปเตอร์ M.2 และไดรฟ์ M.2 ThinkSystem SR665 Lenovo Docs
He saw what a strenuous and complicated it was to. Always add acid to water slowly and with stirring. To prepare a 0.100 m hcl solution from a 1.50 m hcl solution, you need to use the dilution. What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? Pascal.
Letter M Floral Initial Free Stock Photo Public Domain Pictures
He saw what a strenuous and complicated it was to. To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final. In 1 ml of 0.990 m cuso4 solution, there are 0.990 moles of cuso4 present. What volume of.
Ubiquiti NSM2 NanoStation M210.411.2 dBi
What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution? He saw what a strenuous and complicated it was to. Always add acid to water slowly and with stirring. For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12.
Always Add Acid To Water Slowly And With Stirring.
For example, to prepare 1 liter of 0.02 m hcl solution, you would mix 4.2 ml of concentrated hcl (12 m) with 995.8 ml of water. He saw what a strenuous and complicated it was to. To make a 1 molar solution of sucrose, you would weigh out 342.3 grams of sucrose (molecular weight of sucrose is 342.3 g/mol) and dissolve it in water to make a final. Pascal had the idea to invent the calculator while observing and aiding his father's official work as supervisor of taxes at rouen.
In 1 Ml Of 0.990 M Cuso4 Solution, There Are 0.990 Moles Of Cuso4 Present.
To prepare a 0.100 m hcl solution from a 1.50 m hcl solution, you need to use the dilution. What volume of a 1.50 m hcl solution should you use to prepare 2.00 l of a 0.100 m hcl solution?