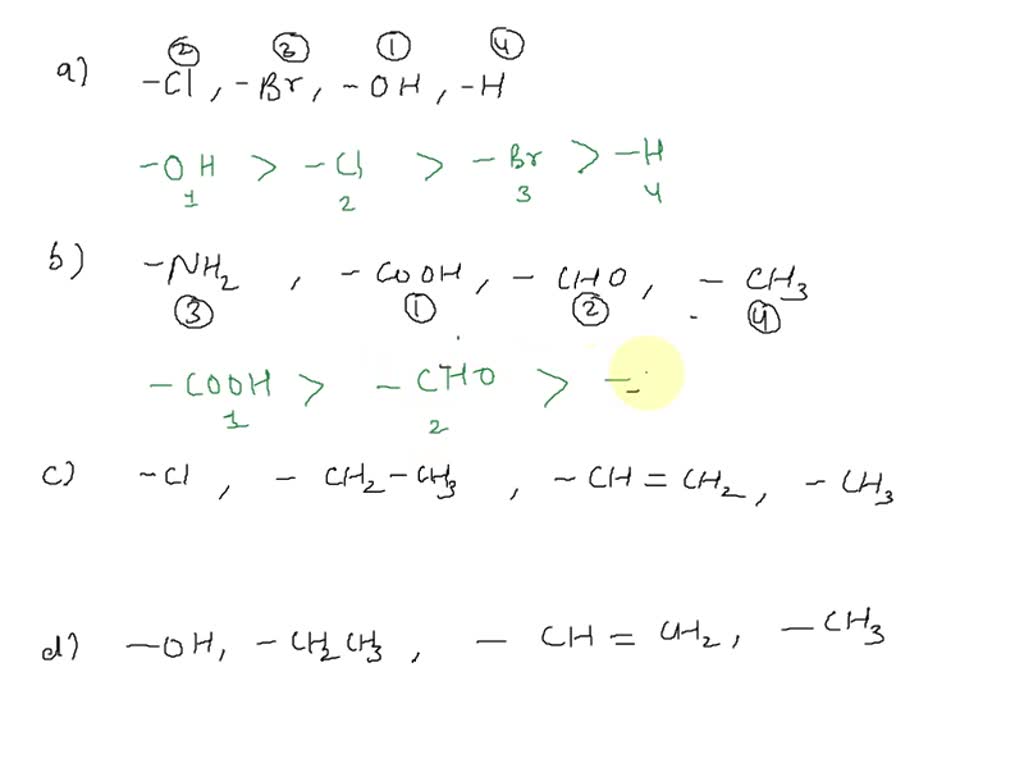Is Ch3 Higher Priority Than Br
Is Ch3 Higher Priority Than Br - In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). Isotopes of higher mass of an element have higher priority. Therefore the two high priority. The ethenyl carbon priority is two. The priority of an atom is first determined by its atomic number, thus i > br > cl > f.
For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). The priority of an atom is first determined by its atomic number, thus i > br > cl > f. The ethenyl carbon priority is two. Therefore the two high priority. Isotopes of higher mass of an element have higher priority. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number.
Therefore the two high priority. Isotopes of higher mass of an element have higher priority. The ethenyl carbon priority is two. For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The priority of an atom is first determined by its atomic number, thus i > br > cl > f.
Why would ch(ch3)2 be in higher priority in r/s than the other groups
Therefore the two high priority. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The ethenyl carbon priority is two. Isotopes of higher mass of an element have higher priority. The priority of an atom is first determined by its atomic number, thus i >.
Why does CH3 have higher priority than H2B? I got this off the key, and
Therefore the two high priority. For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). Isotopes of higher mass of an element have higher priority. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The ethenyl.
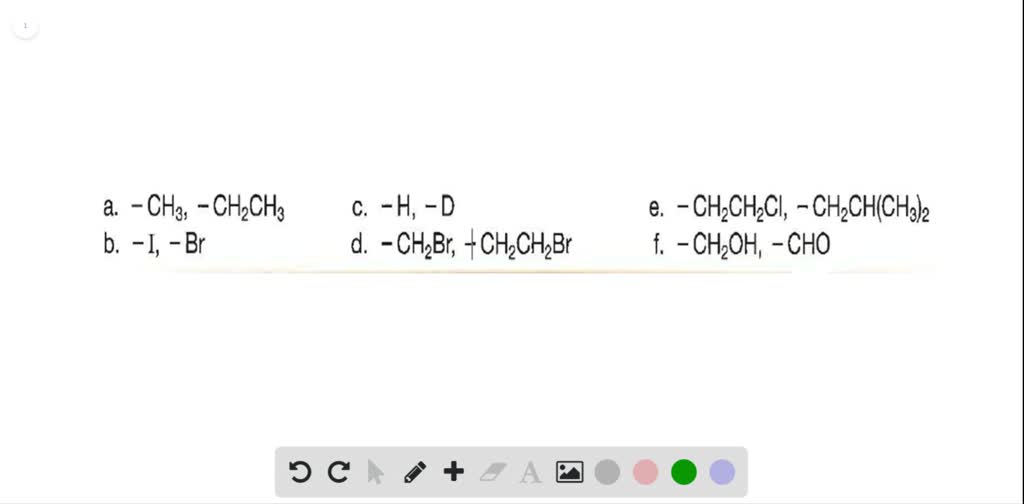
SOLVEDWhich group in each pair is assigned the higher priority? a
Therefore the two high priority. The ethenyl carbon priority is two. For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. Isotopes of higher mass of an.
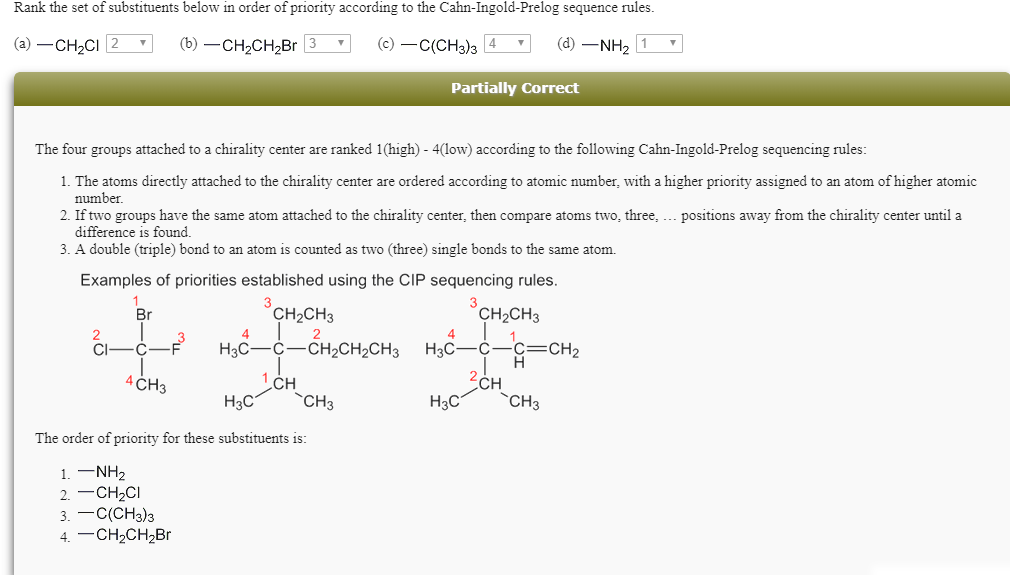
Solved How is C(CH3)3 higher than CH2CH2Br? the third
Isotopes of higher mass of an element have higher priority. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. Therefore the two high priority. The ethenyl carbon priority is two. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1).
SOLVED Which group in each pair is assigned the higher priority? a
Isotopes of higher mass of an element have higher priority. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. Therefore the two high priority. For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). The ethenyl carbon priority is two.
Why does CH3 have higher priority than H2B? I got this off the key, and
Therefore the two high priority. The ethenyl carbon priority is two. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. Isotopes of higher mass of an element have higher priority. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1).
SOLVED Which substitucnt has highcr priority? B. Which substituent has
For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). Therefore the two high priority. Isotopes of higher mass of an element have higher priority. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The ethenyl.
SOLVED Which of the following lists the substituents in the order of
Isotopes of higher mass of an element have higher priority. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The ethenyl carbon priority is two..
Why would ch(ch3)2 be in higher priority in r/s than the other groups
Therefore the two high priority. In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. Isotopes of higher mass of an element have higher priority. The.
Why would ch(ch3)2 be in higher priority in r/s than the other groups
For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). Therefore the two high priority. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. Isotopes of higher mass of an element have higher priority. The ethenyl carbon priority is two.
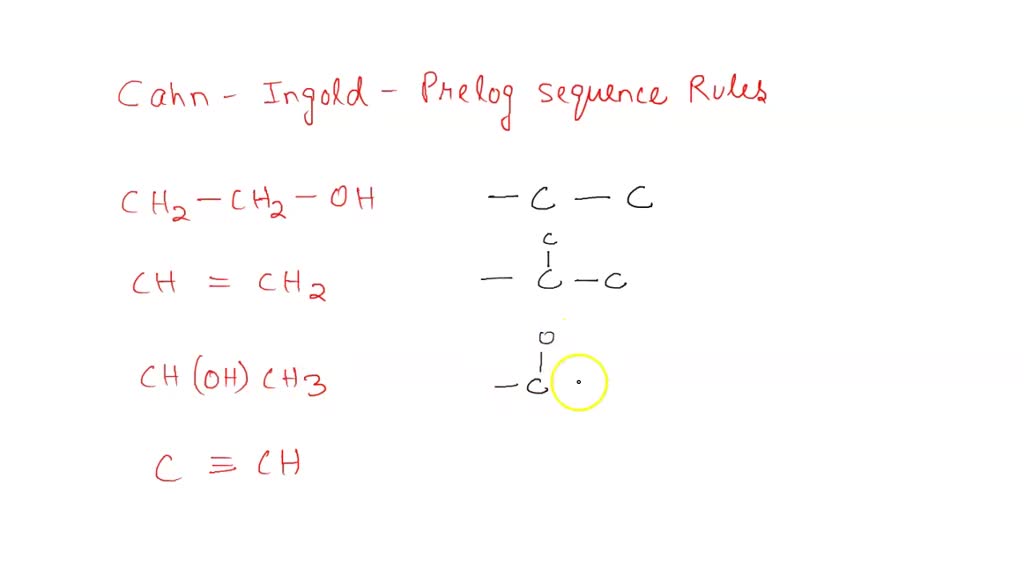
Isotopes Of Higher Mass Of An Element Have Higher Priority.
For example, the ethenyl group (ch 2 =ch) has higher priority than the ethyl group (ch 3 ch 2). In ch3, there are three hydrogens (atomic number 1), and in ch2br, there is one hydrogen (atomic number 1) and one bromine (atomic number. The priority of an atom is first determined by its atomic number, thus i > br > cl > f. The ethenyl carbon priority is two.