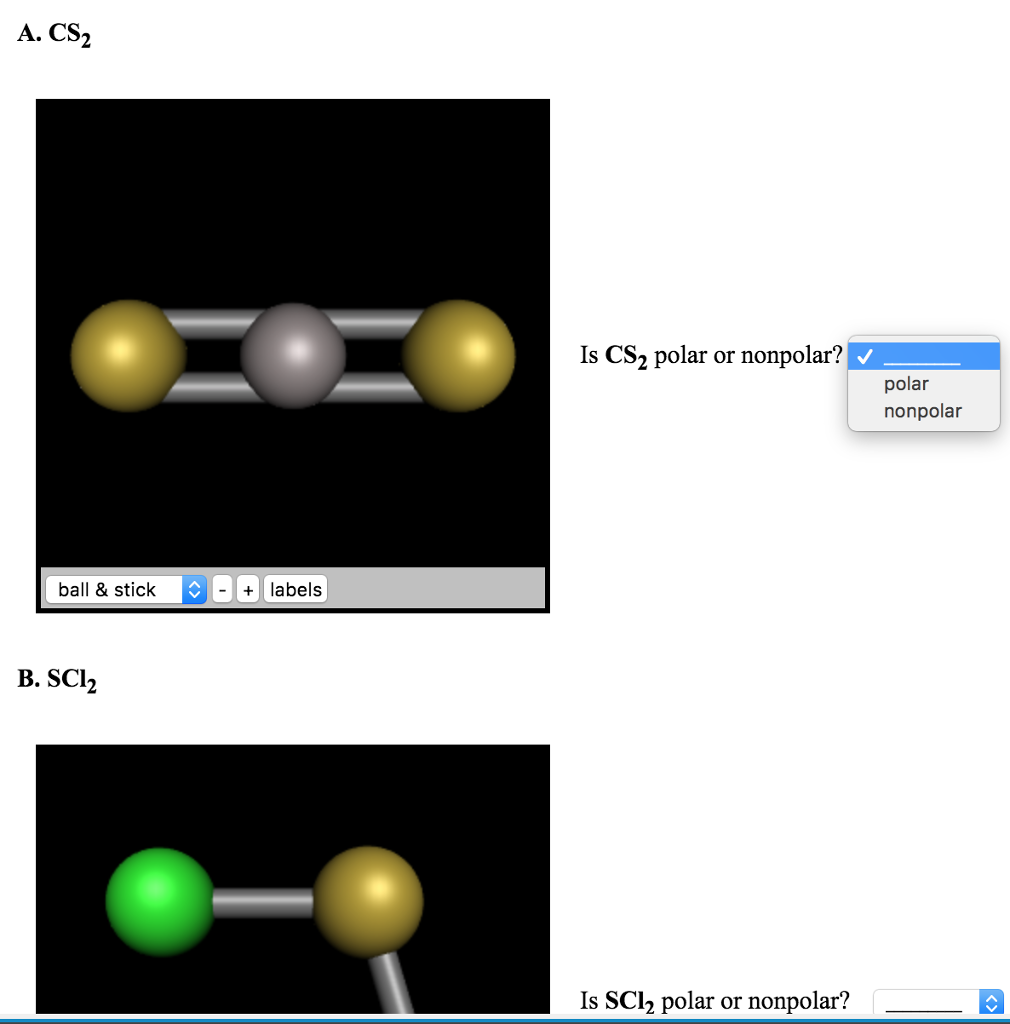
Is Cs2 Polar
Is Cs2 Polar - Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. As a result, cs2 is a nonpolar molecule. Cs2 is cs2 polar or nonpolar? Polar nonpolar ball | chegg.com As a result, cs2 is a nonpolar molecule. The difference in polarity between the two.
Cs2 is cs2 polar or nonpolar? As a result, cs2 is a nonpolar molecule. Polar nonpolar ball | chegg.com Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. The difference in polarity between the two. As a result, cs2 is a nonpolar molecule.
Polar nonpolar ball | chegg.com As a result, cs2 is a nonpolar molecule. The difference in polarity between the two. Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. As a result, cs2 is a nonpolar molecule. Cs2 is cs2 polar or nonpolar?
Ethanol Polar or Nonpolar TianasrHanna
The difference in polarity between the two. Cs2 is cs2 polar or nonpolar? Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. Polar nonpolar ball | chegg.com As a result, cs2 is a nonpolar molecule.
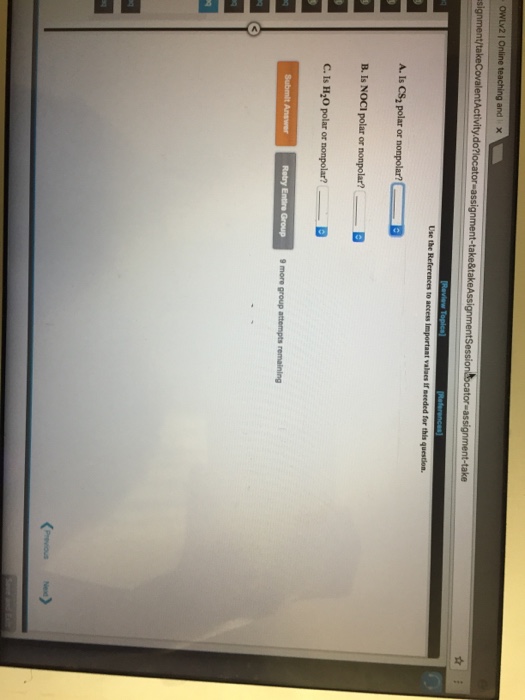
Solved Is CS_2 polar or nonpolar? _______ Is NoCl polar or
Cs2 is cs2 polar or nonpolar? As a result, cs2 is a nonpolar molecule. Polar nonpolar ball | chegg.com Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. The difference in polarity between the two.
Polar Ignite 3 review A midrange running watch for fitness data nerds
Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. As a result, cs2 is a nonpolar molecule. Cs2 is cs2 polar or nonpolar? Polar nonpolar ball | chegg.com As a result, cs2 is a nonpolar molecule.
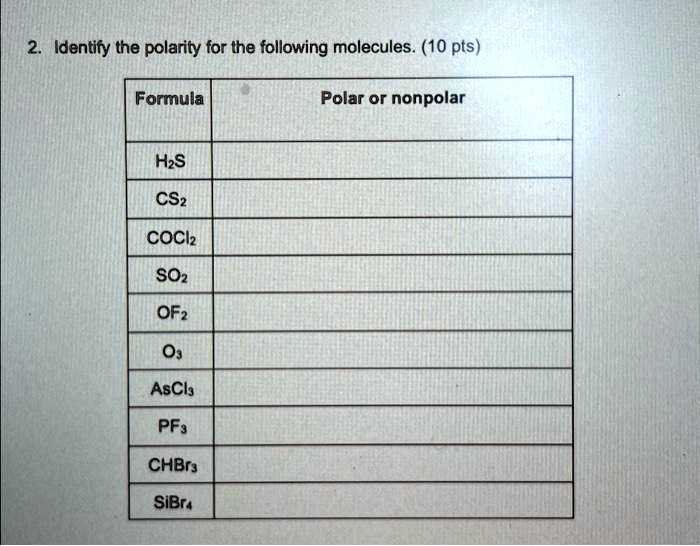
SOLVED 2. Identify the polarity for the following molecules. 10 pts
The difference in polarity between the two. As a result, cs2 is a nonpolar molecule. Polar nonpolar ball | chegg.com Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. Cs2 is cs2 polar or nonpolar?
Scl2 Polar Or Nonpolar Asking List
Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. As a result, cs2 is a nonpolar molecule. Cs2 is cs2 polar or nonpolar? Polar nonpolar ball | chegg.com The difference in polarity between the two.
Is SF2 Polar or Nonpolar? Techiescientist
Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. The difference in polarity between the two. As a result, cs2 is a nonpolar molecule. Polar nonpolar ball | chegg.com As a result, cs2 is a nonpolar molecule.
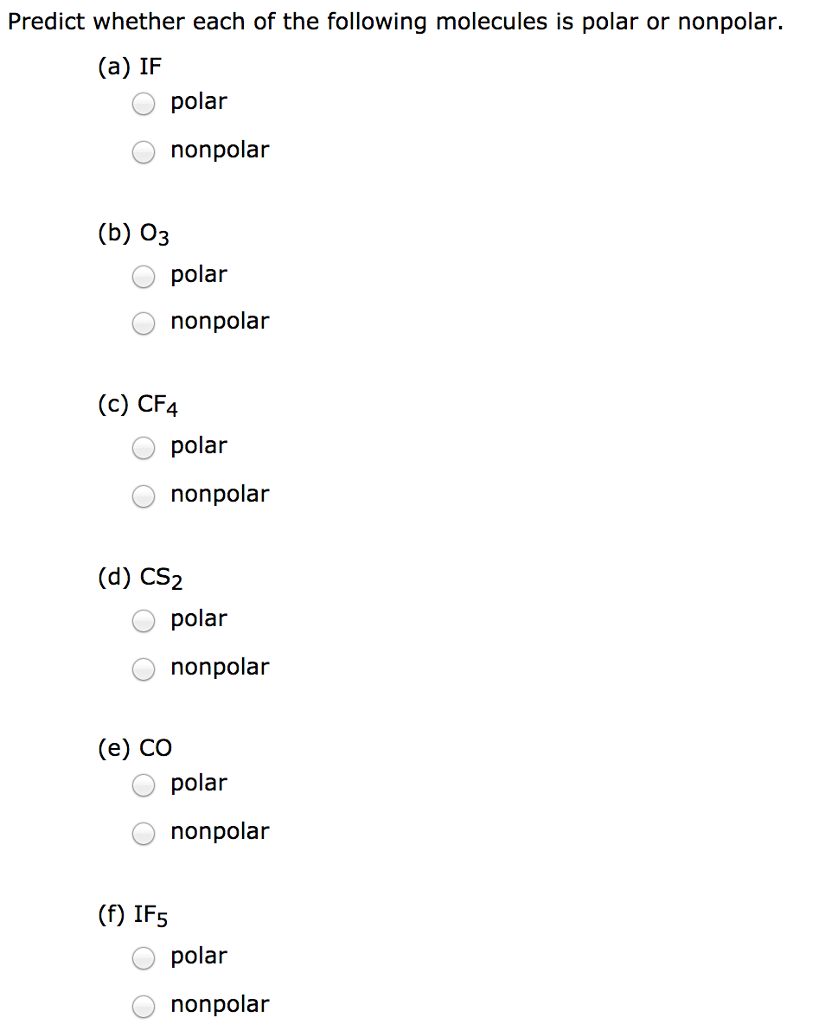
Solved Predict whether each of the following molecules is
Cs2 is cs2 polar or nonpolar? Polar nonpolar ball | chegg.com As a result, cs2 is a nonpolar molecule. Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. The difference in polarity between the two.
Is CS2 Polar or Nonpolar? Polarity of Carbon Disulfide
Cs2 is cs2 polar or nonpolar? Polar nonpolar ball | chegg.com Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. As a result, cs2 is a nonpolar molecule. The difference in polarity between the two.
Is Cs2 Polar Or Nonpolar?
Polar nonpolar ball | chegg.com Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. Cs2 is cs2 polar or nonpolar? The difference in polarity between the two. As a result, cs2 is a nonpolar molecule.
As A Result, Cs2 Is A Nonpolar Molecule.
Yes, carbon disulfide (cs2) is insoluble in water because it is a nonpolar covalent compound and water is a polar solvent. Cs2 is cs2 polar or nonpolar? Polar nonpolar ball | chegg.com The difference in polarity between the two.